Dr Ryan Seipke
- Position
- Associate Professor in Molecular Microbiology
- Areas of expertise
- Streptomyces; natural products; bioinformatics; biocatalysis; bioengineering
- r.seipke@leeds.ac.uk
- Phone
- +44(0)113 343 5604
- Location
- 5.14 Roger Stevens
- Faculty
- Biological Sciences
- School
- Molecular and Cellular Biology
Introduction
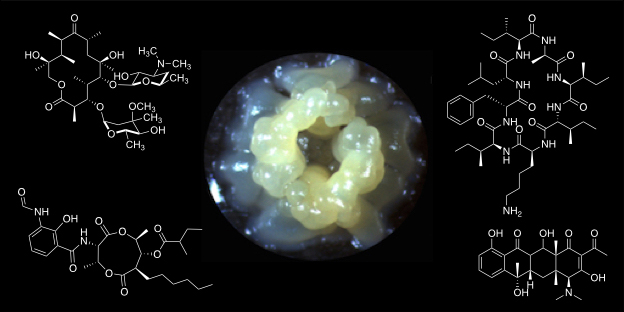
The research group is interested in the biology of Streptomyces bacteria, which are renowned for their diverse and robust secondary metabolism and the production of industrially- and medically-important natural products. Our work focuses on the molecular genetics, genomics and biochemistry of natural product biosynthesis and its regulation. A main goal of ours is to develop robust methods for natural products discovery and bioengineering. We also have a significant interest in understanding the evolution of microbial secondary metabolism and its ecological significance.
Current major projects
- How is secondary metabolism in Streptomyces species regulated?
- How do standalone biosynthetic enzymes from assembly line-like systems work?
Detailed research programme
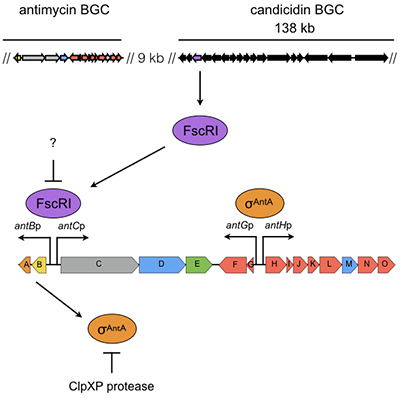
Regulation of antimycin production in Streptomyces species
The majority of the ~30 biosynthetic pathways harboured by an average Streptomyces species are not expressed in the laboratory. Understanding the diversity of regulatory strategies controlling the expression of these pathways is therefore critical if their biosynthetic potential is to be explored for new drug leads. Antimycins are potent inhibitors of the Bcl-2 family of anti-apoptotic proteins, which are frequently overproduced by cancer cells and confer resistance to chemotherapeutic agents whose mode of action is activation of apoptosis. Our goal is to identify factors that control production of antimycins in order to advance our understanding of how the expression of secondary metabolism is controlled and will aid the pursuit of silent biosynthetic pathway activation.
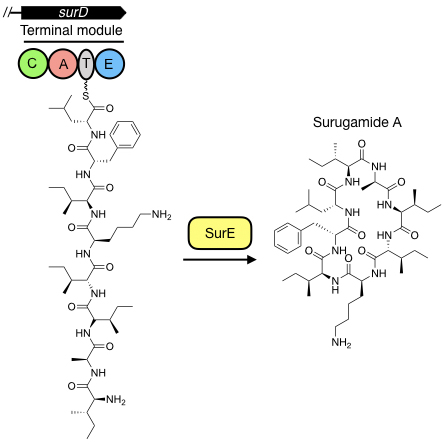
A standalone peptide cyclase that cyclises nonribisomal peptides
The terminal step in the biosynthesis of nonribosomal peptides is the
hydrolytic release and, frequently, macrocyclization of an aminoacyl-S-thioester by
an embedded thioesterase. We have discovered a new family of nonribosomal peptides that are cyclised by a standalone enzyme instead of an embedded thioesterase. Our goal is to understand how this enzyme works in vitro and in vivo using biochemical, biophysical and structural approaches to expand the paradigmatic understanding of how nonribosomal peptides are released from the terminal peptidyl carrier protein.