Pentabromodiphenyl ether
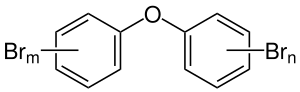 General chemical structure of pentabromodiphenyl ethers, where n + m = 5
| |
| Names | |
|---|---|
| Other names
PentaBDE; Penta-BDE; PeBDE; Penta; PBDPO; Pentabromobiphenyl ether
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.046.425 |
| EC Number |
|
| KEGG |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
| UN number | 3152 3077 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H5Br5O | |
| Molar mass | 564.691 g·mol−1 |
| Appearance | Viscous white to amber-colored liquid[1] |
| Density | 2.25-2.28 g/cm3[1] |
| Melting point | −7 to 3 °C (19 to 37 °F; 266 to 276 K) |
| Boiling point | Decomposes[1] |
| not soluble | |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H362, H373, H410 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
5000 mg/kg (oral, rat)[1] |
| Related compounds | |
Related polybrominated diphenyl ethers
|
octabromodiphenyl ether, decabromodiphenyl ether |
Related compounds
|
diphenylether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pentabromodiphenyl ether (also known as pentabromodiphenyl oxide) is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP).
Composition, uses, and production
[edit]Commercial pentaBDE is a technical mixture of different PBDE congeners, with BDE-47 (2,2',4,4'- tetrabromodiphenyl ether) and BDE-99 (2,2',4,4',5-pentabromodiphenyl ether) as the most abundant.[2] The term pentaBDE alone refers to isomers of pentabromodiphenyl ether (PBDE congener numbers 82-127).[3]
| Structure | Congener | Name | Fraction |
|---|---|---|---|

|
BDE-47 | 2,2′,4,4′-tetra- bromodiphenyl ether |
38–42% |

|
BDE-85 | 2,2′,3,4,4′-penta- bromodiphenyl ether |
2.2–3.0 % |

|
BDE-99 | 2,2′,4,4′,5-penta- bromodiphenyl ether |
45–49% |

|
BDE-100 | 2,2′,4,4′,6-penta- bromodiphenyl ether |
7.8–13 % |

|
BDE-153 | 2,2′,4,4′,5,5′-hexa- bromodiphenyl ether |
5.3–5.4 % |

|
BDE-154 | 2,2′,4,4′,5,6′-hexa- bromodiphenyl ether |
2.7–4.5 % |
Only congeners with more than 1% listed.
Commercial pentaBDE is most commonly used as a flame retardant in flexible polyurethane foam; it was also used in printed circuit boards in Asia, and in other applications.[2] The annual demand worldwide was estimated as 7,500 tonnes in 2001, of which the Americas accounted for 7,100 tonnes, Europe 150 tonnes, and Asia 150 tonnes.[5] The global industrial demand increased from 4,000 tonnes annually in 1991 to 8,500 tonnes annually in 1999.[6] As of 2007, "there should be no current production of C-PentaBDE [commercial pentaBDE] in Europe, Japan, Canada, Australia and the US"; however, it is possible that production continues elsewhere in the world.[2]
Environmental chemistry
[edit]PentaBDE is released by different processes into the environment, such as emissions from manufacture of pentaBDE-containing products and from the products themselves.[2] Elevated concentrations can be found in air, water, soil, food, sediment, sludge, and dust.[2][7][8]
Exposures and health effects
[edit]PentaBDE may enter the body by ingestion or inhalation.[3] It is "stored mainly in body fat" and may stay in the body for years.[3] A 2007 study found that PBDE 47 (a tetraBDE) and PBDE 99 (a pentaBDE) had biomagnification factors in terrestrial carnivores and humans of 98, higher than any other industrial chemicals studied.[9] In an investigation carried out by the WWF, "the brominated flame retardant chemical (PBDE 153), which is a component of the penta- and octa- brominated diphenyl ether flame retardant products" was found in all blood samples of 14 ministers of health and environment of 13 European Union countries.[10]
The chemical has no proven health effects in humans; however, based on animal experiments, pentaBDE may have effects on "the liver, thyroid, and neurobehavioral development."[3]
Voluntary and governmental actions
[edit]In Germany, industrial users of pentaBDE "agreed to a voluntary phaseout in 1986."[11] In Sweden, the government "phase[d] out the production and use of the [pentaBDE] compounds by 1999 and a total ban on imports came into effect within just a few years."[11] The European Union (EU) has carried out a comprehensive risk assessment under the Existing Substances Regulation 793/93/EEC;[12] as a consequence, the EU has banned the use of pentaBDE since 2004.[13]
In the United States, as of 2005, "no new manufacture or import of" pentaBDE and octaBDE "can occur... without first being subject to EPA [i.e., United States Environmental Protection Agency ] evaluation."[14] As of mid-2007, a total of eleven states in the U.S. had banned pentaBDE.[15]
In May 2009, pentaBDE was added to the Stockholm Convention as it meets the criteria for the so-called persistent organic pollutants of persistence, bioaccumulation and toxicity.
Alternatives
[edit]The EPA organized a Furniture Flame Retardancy Partnership beginning in 2003 "to better understand fire safety options for the furniture industry" after pentaBDE "was voluntarily phased out of production by the sole U.S. manufacturer on December 31, 2004."[16] In 2005 the Partnership published evaluations of alternatives to pentaBDE, including triphenyl phosphate, tribromoneopentyl alcohol, tris(1,3-dicholoro-2-propyl)phosphate, and 12 proprietary chemicals.[17]
References
[edit]- ^ a b c d Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ^ a b c d e Persistent Organic Pollutants Review Committee of the Stockholm Convention. Commercial Pentabromodiphenyl Ether: Risk Management Evaluation. United Nations Environment Programme, August 2007.
- ^ a b c d Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polybrominated Biphenyls and Polybrominated Diphenyl Ethers (PBBs and PBDEs). Archived 2007-10-31 at the Wayback Machine Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service, September 2004.
- ^ M. J. La Guardia, R. C. Hale, E. Harvey: Detailed Polybrominated Diphenyl Ether (PBDE) Congener Composition of the Widely Used Penta-, Octa-, and Deca-PBDE Technical Flame-retardant Mixtures, Environ. Sci. Technol., 2006, 40, 6247–6254, doi:10.1021/es060630m.
- ^ Bromine Science and Environmental Forum. Major Brominated Flame Retardants Volume Estimates: Total Market Demand By Region in 2001. Archived 2006-11-30 at the Wayback Machine 21 January 2003.
- ^ Dynamic Substance Flow Analysis Model for Selected Brominated Flame Retardants as a Base for Decision Making on Risk Reduction Measures, study for the Swiss National Science Foundation, 2007, page 23
- ^ Hale RC, La Guardia MJ, Harvey E, Gaylor MO, Mainor TM (2006): Brominated flame retardant concentrations and trends in abiotic media. Chemosphere. 64(2):181-6. doi:10.1016/j.chemosphere.2005.12.006 PMID 16434082
- ^ Stapleton, Heather M., et al. Polybrominated Diphenyl Ethers in House Dust and Clothes Dryer Lint.] Environmental Science & Technology 39(4), 925-931, 2005. doi:10.1021/es0486824
- ^ Kelly, Barry C., et al. Food Web–Specific Biomagnification of Persistent Organic Pollutants. Science 13 July 2007: Vol. 317. no. 5835, pp.236-239.
- ^ WWF Detox Campaign. Bad Blood? A Survey of Chemicals in the Blood of European Ministers. October 2004.
- ^ a b Alcock, R. E. and J. Busby (2006): Risk migration and scientific advance: The case of flame-retardant compounds. Risk Analysis 26(2): 369-381. doi:10.1111/j.1539-6924.2006.00739.x PMID 16573627
- ^ European Union risk assessment report. Diphenyl ether, pentabromo derivative (pentabromodiphenyl ether). Archived 2007-03-17 at the Wayback Machine Luxembourg: Office for Official Publications of the European Communities, 2001. Publication EUR 19730 EN. ISBN 92-894-0479-5
- ^ Directive 2003/11/Ec of the European Parliament and of the Council of 6 February 2003 amending for the 24th time Council Directive 76/769/EEC relating to restrictions on the marketing and use of certain dangerous substances and preparations (pentabromodiphenyl ether, octabromodiphenyl ether). Official Journal of the European Union 15.2.2003.
- ^ U.S. Environmental Protection Agency. Polybrominated diphenylethers (PBDEs). "Last updated on Thursday, August 2nd, 2007." Accessed 2007-10-26.
- ^ Maine Joins Washington, Bans PBDEs. Archived 2007-08-02 at the Wayback Machine Washington, DC: National Caucus of Environmental Legislators, June 18, 2007.
- ^ EPA. Furniture Flame Retardancy Partnership page. "Last updated on Monday, September 18th, 2006." Accessed 2007-10-31.
- ^ EPA. Environmental Profiles of Chemical Flame-Retardant Alternatives for Low-Density Polyurethane Foam. Volumes 1 and 2. September 2005.