Asupre
 | ||||||||||||||||||||||||||||||||||||
| Sulfur | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alternative name | Sulphur (British spelling) | |||||||||||||||||||||||||||||||||||
| Allotropes | tingnan ang mga alotropo ng asupre | |||||||||||||||||||||||||||||||||||
| Appearance | Lemon yellow sintered microcrystals | |||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(S) | ||||||||||||||||||||||||||||||||||||
| Sulfur sa talahanayang peryodiko | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| Atomikong bilang (Z) | 16 | |||||||||||||||||||||||||||||||||||
| Group | 16 | |||||||||||||||||||||||||||||||||||
| Period | 3 | |||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p4 | |||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 6 | |||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||
| Phase at STP | solido | |||||||||||||||||||||||||||||||||||
| Melting point | alpha (α-S8): 388.36 K (115.21 °C, 239.38 °F) | |||||||||||||||||||||||||||||||||||
| Boiling point | 717.8 K (444.6 °C, 832.3 °F) | |||||||||||||||||||||||||||||||||||
| Density (near r.t.) | alpha (α-S8): 2.07 g/cm3 beta (β-S8): 1.96 g/cm3 gamma (γ-S8): 1.92 g/cm3 | |||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 1.819 g/cm3 | |||||||||||||||||||||||||||||||||||
| Critical point | 1314 K, 20.7 MPa | |||||||||||||||||||||||||||||||||||
| Heat of fusion | beta (β-S8): 1.727 kJ/mol | |||||||||||||||||||||||||||||||||||
| Heat of vaporization | beta (β-S8): 45 kJ/mol | |||||||||||||||||||||||||||||||||||
| Molar heat capacity | 22.75 J/(mol·K) | |||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||
| Oxidation states | −2, −1, 0, +1, +2, +3, +4, +5, +6 (isang matapang na asidikong oksido) | |||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.58 | |||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||
| Covalent radius | 105±3 pm | |||||||||||||||||||||||||||||||||||
| Van der Waals radius | 180 pm | |||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordiyal | |||||||||||||||||||||||||||||||||||
| Crystal structure | alpha (α-S8): orthorhombic (oF128) | |||||||||||||||||||||||||||||||||||
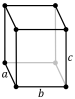
| Lattice constants | a = 1.0460 nm b = 1.2861 nm c = 2.4481 nm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||
| Crystal structure | beta (β-S8): monoclinic (mP48) | |||||||||||||||||||||||||||||||||||
| Lattice constants | a = 1.0923 nm b = 1.0851 nm c = 1.0787 nm β = 95.905° (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||
| Thermal conductivity | 0.205 W/(m⋅K) (amorphous) | |||||||||||||||||||||||||||||||||||
| Electrical resistivity | 2×1015 Ω⋅m (at 20 °C) (amorphous) | |||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[4] | |||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | alpha (α-S8): −15.5×10−6 cm3/mol (298 K)[5] | |||||||||||||||||||||||||||||||||||
| Bulk modulus | 7.7 GPa | |||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.0 | |||||||||||||||||||||||||||||||||||
| CAS Number | 7704-34-9 | |||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||
| Discovery | before 2000 BCE[6] | |||||||||||||||||||||||||||||||||||
| Recognized as an element by | Antoine Lavoisier (1777) | |||||||||||||||||||||||||||||||||||
| Isotopes of sulfur | ||||||||||||||||||||||||||||||||||||
34S abundances vary greatly (between 3.96 and 4.77 percent) in natural samples. | ||||||||||||||||||||||||||||||||||||
Ang asupre, sangyawa o sulpura (Kastila: azufre, Ingles: sulfur) ay isang kemikal na elementong gumagamit sa bilang atomikong 16.[7] Ito ay karaniwang ginagamitan ng simbolong S. Ito ay isang karaniwang multibalente na hindi metal. Asupre, sa katutubong uri, ay matamlay na dilaw na crystal line solid. Sa kalikasan, karaniwan ito matatagpuang purong elemento at mga mineral na asuprido at asuprata. Isa itong esensyal na elemento para sa buhay at matatagpuan ito sa mga asidong amino: cysteine at methionine. Ginagamit itong pataba, pero karaniwan din ito sa pulbura, posporo, insecticide at fungicide. Ang elementong asupre na kristal ay kadalasang hinahanap ng mga nangongolekta ng mga mineral dahil sa matingkad nitong mga sukat na polihedron. Ito rin ay kilala sa pantanggal ng mga libag sa mga sabong may kahalong sulfur. Tinatawag din itong brimstone sa Inggles.
Mga sanggunian
[baguhin | baguhin ang wikitext]- ↑ "Standard Atomic Weights: Sulfur" (sa wikang Ingles). CIAAW. 2009.
{{cite web}}: CS1 maint: date auto-translated (link) - ↑ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry (sa wikang Ingles). doi:10.1515/pac-2019-0603. ISSN 1365-3075.
{{cite journal}}: CS1 maint: date auto-translated (link) - ↑ 3.0 3.1 Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
{{cite book}}: CS1 maint: date auto-translated (link) - ↑ Lide, D. R., pat. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (ika-86th (na) edisyon). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
{{cite book}}: CS1 maint: date auto-translated (link) - ↑ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
{{cite book}}: CS1 maint: date auto-translated (link) - ↑ "Sulfur History". Georgiagulfsulfur.com. Nakuha noong 2022-02-12.
{{cite web}}: CS1 maint: date auto-translated (link) - ↑ Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics (PDF). CRC press. 2000. ISBN 0849304814.
{{cite book}}: CS1 maint: date auto-translated (link)
| Talahanayang peryodiko | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | He | ||||||||||||||||||||||||||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||||||||||||
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||||||||||||
| Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | ||||||||||
| Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | ||||||||||
|
|||||||||||||||||||||||||||||||||||||||||